Each SARS-CoV-2 virion consists of 4 structural proteins: S (spike protein), E (envelope), M (membrane), and N (nucleocapsid). Originally designed for use in serum testing and employed in all phase III clinical trials funded by Operation Warp Speed, this SARS-CoV-2 assay effectively tracks antibody responses to SARS-CoV-2 vaccines or natural SARS-Cov-2 infections over time and determines comparative antibody levels by including a reference standard. By adapting and optimizing the MSD serum antibody assay for use with saliva, samples self-collected at home using Salimetrics easy-to-use saliva collection kits can be shipped to a central site for testing. Home collection provides participants with a simple solution that increases willingness to collect several samples over time to track responses, an important factor in vaccine efficacy.
Current research has shown that antibody levels decrease in the serum of COVID-19 patients over time (4) and vary depending on disease severity.
Technical Summary
| Panel Summary | |
|---|---|
| Optimum Collection Volume: | 100 μL |
| Total Number of Samples Required: | 1 |
| Methodology: | ECL |
| Target SARS-CoV-2 Antibodies |
|---|
| SARS-CoV-2 Spike Protein (S1) SARS-CoV-2 Nucleocapsid Protein (N) SARS-CoV-2 Receptor Binding Domain (RBD) |
| Assay Performance* | |
|---|---|
| Sensitivity: | 96.5% |
| Specificity: | 98.8% |
| Predictive Value (at 5% Prevalance)* | |
|---|---|
| PPV: | 81% |
| NPV: | 99.8% |

How to collect Salivary SARS-CoV-2 (IgG) Antibody Panel
APPROVED SARS-CoV-2 IgG SALIVA COLLECTION METHODS
SARS-CoV-2-Specific Antibody Responses in Saliva
By testing for coronavirus antibodies to a combination of SARS-CoV-2 antigens (Nucleocapsid Protein, Spike Protein, or Receptor Binding Domain in Spike) in the same saliva sample, this test can determine if a subject is:
| Status of Individual | Assay Results |
|---|---|
| Uninfected | N, Spike and RBD negative |
| Infected naturally | N, Spike and RBD positive |
| Vaccinated | N negative, Spike and RBD positive |
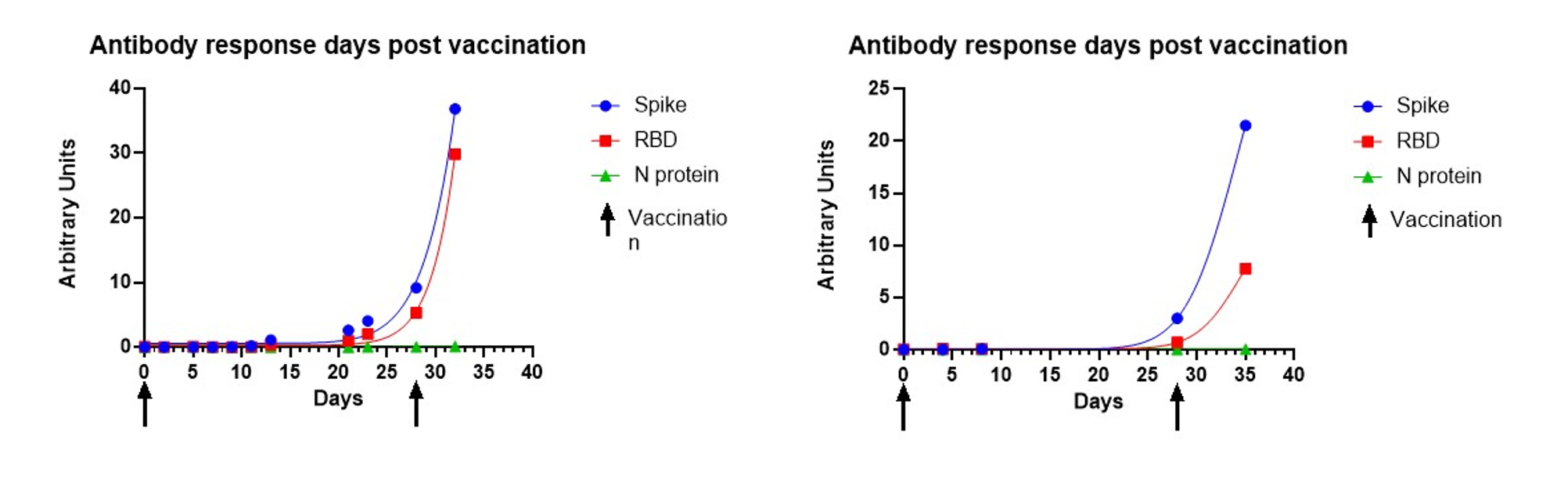
This assay can also effectively track the antibody response to vaccine or natural infection over time and provide relative levels by including a reference standard. Below is representative performance data of an individual sampled by passive drool from the first day of immunization with the Moderna vaccine through a week after receiving their second dose of the vaccine. The lack of N-protein reactivity (shown in green) distinguishes the SARS-CoV-2 vaccination, since the vaccine does not encode N-protein. Here, you can see a moderate increase in Spike Protein/RBD reactivity up to the second immunization (indicated by arrow) and the exponential increase in reactivity post second immunization.
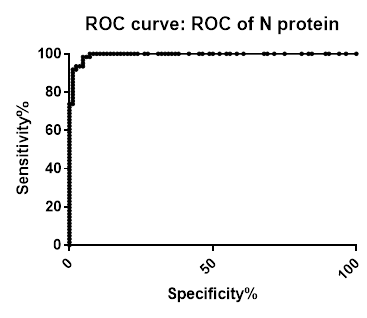
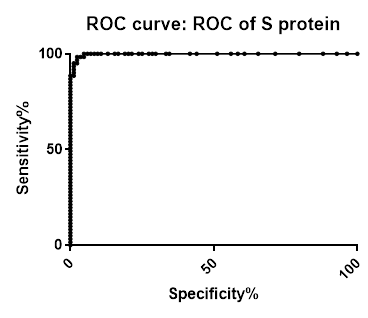
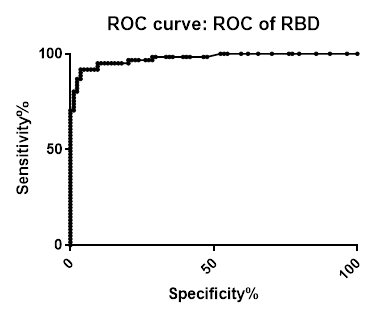
ROC analysis summary
ROC analysis summary for SARS-CoV-2 IgG Antibody Assay, 3-Plex (S-Protein, N-Protein, & RBD) Panel on Passive Drool samples pre-COVID-19 and PCR confirmed positive
| N-Protein | Spike | RBD | |
|---|---|---|---|
| Area under the ROC curve | |||
| Area | 0.994 | 0.9977 | 0.9762 |
| Std. Error | 0.00396 | 0.001899 | 0.01112 |
| 95% confidence interval | 0.9862 to 1.002 | 0.9939 to 1.001 | 0.9544 to 0.9980 |
| P value | < 0.0001 | < 0.0001 | < 0.0001 |
| Data | |||
| Controls (Negative N-protein) | 84 | 84 | 84 |
| Patients (Positive N-protein) | 61 | 61 | 61 |
 |
 |
 |
Note: Salimetrics is currently accepting both serum and saliva research samples for COVID-19 antibody testing. Detection of anti-SARS-CoV-2 IgA and IgM are also available upon request.
References & SARS-CoV-2 (IgG) Antibody Research
- Hoepel, et al., (2020). Anti-SARS-CoV-2 IgG from severely ill COVID-19 patients promotes macrophage hyper-inflammatory responses. bioRxiv.
- Johnson, et al., (2020). Evaluation of a novel multiplexed assay for determining IgG levels and functional activity to SARS-CoV-2. Clinical Virology.
- Grandjean, et al., (2020). Humoral Response Dynamics Following Infection with SARS-CoV-2. medRxiv.
- Corbett, et al., (2020). Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. The New England Journal of Medicine.
- Folegatti, et al., (2020). Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet.
- Pollock, et al., (2021). Correlation of SARS-CoV-2 nucleocapsid antigen and RNA concentrations in nasopharyngeal samples from children and adults using an ultrasensitive and quantitative antigen assay. Journal of Clinical Microbiology.
- Majdoubi, et al., (2020). Antibody reactivity to SARS-CoV-2 is common in unexposed adults and infants under 6 months. medRxiv.
- Diorio, et al., (2020). Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS–CoV-2. The Journal of Clinical Investigation.
- Huang, et al., (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet.
- Channappanvar R, Perlman S., (2017). Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopatholology.
- Li et al., (2020). Coronavirus infections and immune responses. Journal of Medical Virology.
- Handbook of Covid-19 Prevention and Treatment.
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)